Abstract
This review for the first time aims to investigate the effect of silanization on the ceramic membrane distillation (CMD) as a promising thermally driven separation process. In this regard, the effects of three main factors, namely silane concentration, silane duration, and time as well as the important operating parameters on the process were studied. At the end of this work, future challenges and recommendations in the CMD have also been addressed. The literatures have confirmed that silanization of the CM surface, causes significant changes in the membrane structure in terms of hydrophobicity (water contact angle > 130°), creating different functional groups on the surface and improving the efficiency of the process. The results of previous research woks indicate that the best conditions for the silanization process are possible at silanization time (about 72 h), the number of grafting cycles (1–5 times), and silane concentration (2 or 10 wt%). Concluding the results of various studies shows that the efficiency of the MD for desalination process was high (removal > 98%) under certain conditions (feed input temperature: 70–80 °C and flow rate of 0.3–400 L/h).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lack of fresh water with low water quality because of both natural such as lithological, climatic, atmospheric, hydrological, and topographical factors (Uddin et al. 2021) as well as human-related activities, for instance, livestock farming, mining, waste production and disposal, and so on (Uddin et al. 2021) due to the population growth, improvement of living standards, agricultural sector, prosperity of the industrialization has been considered as a major challenge in the worldwide. It is estimated that more than one billion people on earth do not have access to fresh and clean water (Mishra et al. 2021). The total volume of fresh water reservoirs may be sufficient to meet the current demand, but unfortunately the distribution of these reservoirs does not correspond to the distribution of the population around the world (Gleick 1993; Shirazi et al. 2012). So, because of decreasing the water resources, it is imperative to focus on the water scarcity solutions specially using reusing of treated wastewater or water desalination to improve water supply and sanitation (Van Vliet et al. 2021).
As mentioned earlier, the research for the new water purification process is increasing dramatically, so that among the various purification processes, water desalination has been deemed as a clean process which has been using brackish or seawater to produce clean water with low pollution production (Lattemann and Höpner 2008; Elimelech and Phillip 2011). As an alternative to first-generation thermal desalination techniques, second-generation desalination technologies based on membrane treatment (mainly reverse osmosis (RO)) have gained popularity over the last two decades (Greenlee et al. 2009). It is well known that there are three main technologies extensively applied in water desalination, including reverse osmosis (RO), thermal evaporation (TE), and membrane distillation (MD) (Fan et al. 2017). Among which, although RO is specific technique for water desalination because of its outstanding advantages especially high separation efficiency, its application is limited due to various operational issues such as increased osmotic pressure with increased feed concentration, low recovery ratio, and brine production (Pagliero et al. 2020).
So, up to date, membrane distillation process is a promising and alternative method for purification of water compared to high pressure membrane processes because of using low-grade heat sources (Parani and Oluwafemi 2021). Generally, MD process is one of the emerging membrane separation processes, which refers to the thermal transfer of vapors through a non-wetting porous hydrophobic membrane, the driving force of which is the vapor pressure difference between the two sides of the membrane pores (Parani and Oluwafemi 2021). This technology has many advantages, for example, lower fouling properties, cost-efficient process, has a lower operating temperatures, unlimited osmotic pressure, high permeation purity, lower operating costs, high salt removal, and lower the hydrostatic pressures compare with the pressurized processes (microfiltration (MF) membranes, nanofiltration (NF), ultrafiltration (UF), RO) especially during water treatment (Kujawa et al. 2014; Bandar et al. 2021; Omar et al. 2022; Zhang et al. 2023).
The membrane distillation process, which was registered the first invention of that by Budel on June 3, 1963, unlike the other mentioned methods, is a non-isothermal process that has been known for more than forty years, but in fact it still needs to be developed for its industrial implementation (Budel 1963). In this process, the low surface energy of the hydrophobic MD membrane prevents the pores from getting wet by water penetration, so that water evaporates across the MD membrane from the hot side to the cold side, which is caused by the vapor pressure difference (Fan et al. 2017).
In general, the microporous membrane with high hydrophobicity in all MD processes can be divided into several categories, including vacuum membrane distillation (VMD), swept gas membrane distillation (SGMD), direct contact membrane distillation (DCMD), and air gap membrane distillation (AGMD) (Ren et al. 2017) so that these membranes not only act as a physical barrier to separate the feed solution and seepage due to its hydrophobic nature to determine the salt excretion, but also play an important role in the migration and diffusion of vapor molecules to determine the permeate flux (Ren et al. 2017). Each of these processes has advantages and disadvantages discussed in Table 1 (Ding et al. 2006; Summers and Arafat 2012).
It is better to mention that some crucial characteristics in the fabrication of MD membranes must be considered. These characteristics consist of high liquid inlet pressure (LEP), low thermal conductivity, high hydrophobicity, sharp pore size distribution, having a low surface energy in order to avoid membrane wetting, high thermal mechanical, high porosity, high fouling resistance, low thickness, low torsion to increase water penetration, low thermal conductivity, and as well as chemical stability to increase useful life are necessary to achieved in MD for the high performance (Ren et al. 2017; Ko et al. 2018). On the other hand, superhydrophobic membrane typically shows high static contact angle of > 150° as well as low roll-off angle (less than 10°) at its surface (Bhushan and Her 2010).
In general, membranes can be fabricated from two main groups materials; organic materials such as polymers and also inorganic materials (ceramic) (Yun et al. 2015). The most common membranes used in water and wastewater treatment are polymer structures such as cellulose acetate (CA), polysulfone (PS), and polyether sulfone (PES). However, some limitations of such membranes for stable performance are frequent testing, repair, and replacement of organic polymer membrane due to lower mechanical strength and also chemical stability (Yun et al. 2015). On the other hand, MD membranes must be hydrophobic and porous, which allows water vapor to pass through. Currently, polymer membranes such as polypropylene (PP), polytetrafluoroethylene (PTFE), and polyvinylidene fluoride (PVDF) are commonly used for MD applications. But polymeric membranes are sensitive to high temperature and membrane tendency to fouling phenomena caused by the existence of microorganisms in seawater, which leads to decrease the specific life-span of these types of membranes (Zhang et al. 2014).
To tackle the above issue of organic membranes, recently, researchers have been focused on another category of membranes which is called ceramic membranes because of their mechanical and thermal stability, chemical stabilities, high flux, and are mostly fabricated from metal oxides (such as silica, alumina, and zirconia). Although they show better performance compared to the other types of membranes, the presence of hydroxyl groups (–OH) on the surface of the ceramic membrane leads to its hydrophilic nature (Picard et al. 2001; Krajewski et al. 2006; Hubadillah et al. 2019a; Zhang et al. 2023) and limits the application for CMD. For this reason, hydrophilic ceramic membrane is undesirable for direct use in membrane distillation (Wang et al. 2019).
One of the most important requirements in the MDs processes is the usage of hydrophobic membranes, but since all ceramic membranes have a hydrophilic surface, it is necessary to modify their surface. For this reason, many techniques such as the use of silane agents such as alkyl silane coupling, or fluoroalkylsilane, perfluorodecyltriethoxysilane, trimethylchlorosilane, or trichloromethylsilane (Hendren et al. 2009; Chen et al. 2018b), modification using plasma (Kujawa et al. 2017), microwave plasma chemical vapor deposition (MWPE-CVD), anodic oxidation of aluminum (Kujawa et al. 2016) have been used to change membrane properties toward hydrophobicity, all with the aim of increasing the contact angle (CA), and therefore water pressure will be increased (Pagliero et al. 2020). On the other hand, the use of the above overmentioned methods can be affective to change the morphology of the membranes because the linking molecules can polymerize and create a thin layer that reduces the size of the pores or even closes some of the pores and as a result of that porosity of the membrane reduces (Pagliero et al. 2020). Therefore, it is necessary to choose a hydrophobic material to modify the surface. It should also be noted that in the case of porous ceramic materials, modification using materials with low surface energy is the most common method so that various studies over the years have used silane agents to modify the surface of ceramic membranes from hydrophilic to hydrophobic. For instance, Khemakhem and Amar (2011) conducted experiments on the modification of clay-based ceramic microfiltration membrane using the silane agent of triethoxy-1H,1H,2H,2H-perfluorodecylsilane, and the results of the study showed that after the membrane modification, the obtained contact angle was 141 degrees. It was so that this membrane was able to show a high efficiency (93%) in salt removal with a concentration of 0.1 M and a flux of 17 kg/m2 h (Khemakhem and Amar 2011).
In the MD process with the aforementioned methods, various parameters have effect on its efficiency, which can depend on the selection of type, silane dosage, the duration of the silanization process, the inlet temperature to the membrane distillation process, the inlet salt concentration, and the flow rate, therefore, optimizing the mentioned parameters plays critical role in the efficiency of the MD process. Due to the importance of this issue, in this review article, for the first time, an attempt has been made to study the effects of the aforementioned factors. Also, the authors have tried to discuss the effect of different silane agents in terms of functional groups and its dosage on the physical and chemical properties (such as CA, morphology, pore size, and porosity) of the fabricated ceramic membrane. Besides, a comprehensive review of various studies conducted on different membrane processes and the effects of various parameters, especially inlet temperature, salt concentration, and flow rate on membrane efficiency in terms of flux and salt removal have also been compared. Finally, it has been tried to evaluate the perspectives and recommending some future challenges of using hydrophobic ceramic membranes for desalination processes.
Salinization of ceramic membrane
Type of silane
Nowadays, various organosilane agents with unique functional groups have been used to create a specific surface charge on the membrane for the modification of ceramic membranes surface and also increasing their hydrophobic properties (Lee et al. 2016). Silane agents are molecules in which one Si atom is bonded to four functional groups (SiX4). Moreover, organosilane is the most useful classification of silane agents that consists of at least one carbon–silicon bond structure (CH3–Si–). This bond is very stable and nonpolar, which causes a low surface energy and creates a hydrophobic property on the surface of a CMs after bonding (Mizoshita et al. 2011). The structures of silicon hydride (–Si–H) and reactive substituent (–Si–OCH3) exhibit high reactivity. The combination of carbon–carbon double bonds with polar solvents results in the formation of active silanol species (–Si–OH). New substances composed of silicon and carbon are produced as a result of these reactions.
It is better to note that the chemistry of the functional organic group is influenced by the type of methoxy group attached to the carbon compound, which in turn affects the behavior of the silicon atom. If the group separating the organic compounds is a propylene bond (for example, –CH2CH2CH2–), the reaction of organosilane will function in a similar way to the reactions of the organic compounds in carbon chemistry (Ahmad et al. 2015).
The process of functionalizing the surface of CMs using various functional groups with low surface free energy, for example, fluorine-containing compounds such as fluorinated alkyl silanes (FAS), is often used for changing the hydrophobicity of ceramic surfaces properties (Kujawa et al. 2016). In this case, FAS modifiers are bonded strongly to the surface of the ceramic membrane, which contains a significant number of hydroxyl groups (Kujawa et al. 2016).
In the recent years, various FAS compounds include 1H,1H,2H,2H-perfluorodecyltriethoxysilane, perfluorodecyltrichlorosilane and hexadecyltrimethoxysilane have been used for hydrophobizing the surface of ceramic membranes (Zhou et al. 2020). The presence of 4 to 10 carbon atoms in fluoroalkylsilanes results in a significant concentration of –CF3 and –CF2 groups. These can be incorporated in materials to decrease surface energy (Zhou et al. 2020). However, a chemical called 1H,1H,2H,2H-perfluorooctyltrichlorosilane not only has more –CF2 groups, but also has a longer molecule structure (Zhou et al. 2020), so it has a lower ability to stick to surfaces than other fluorocarbons. Incorporating perfluorinated silanes as water-resistant agents in the manufacturing of foamed ceramic walls can considerably augment their roughness by allowing ample fluoropolymer to occupy the spaces between particles (Zhou et al. 2020). However, as mentioned before, the use of these substances depends on the reason of using them and the main goal is to make the membranes which have surface with an electric charge (Lee et al. 2016). For instance, one substance called trimethoxy(propyl)silane has a chemical structure with a methyl group (–CH3). It can make the surface of the membrane without any specific charge (no positive or negative charge) by sticking to the membrane (Lee et al. 2016). And another examples of silane agents that can create various surface charge on the membrane are (3-aminopropyl)triethoxysilane and 3-(trihydroxysilyl)-1-propanesulfonic acid. In the first compound because of the presence of amine group the surface of CM will be changed to positive charge and in the second compound due to the existence of sulfonic group, a negative charge on the surface of the membranes will be created (Lee et al. 2016).
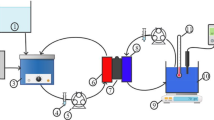
Another classification of silane agents is based on the number of carbon atoms in the chain, for example, perfluoroalkylsilane (PFAS) compounds can be divided into 4 general categories; 6-carbon (1H,1H,2H,2H-perfluorooctyltriethoxysilane), 8-carbon (H,1H,2H,2H-perfluorodecyltriethoxysilane), 10-carbon (1H,1H,2H,2H-perfluorododecyltriethoxysilane), and 12 carbon (1H,1H,2H,2H-perfluorotetradecyltriethoxysilane) (Kujawa et al. 2016). In general, the process of modifying the surface of the ceramic membrane using silane agents is called the grafting process, which is done through the reaction between the hydroxyl groups (–OH) present on the surface of the ceramic membrane and the active groups (ethoxy, methoxy, and chlorine atoms) of organosilane compounds (Aloulou et al. 2021). Because of grafting process stable covalent bonds will be formed and as a result of that when ceramic membrane is treated with organosilane compounds, a very thin layer is formed on the surface and this layer enhances the water resistance of the ceramic membranes (Aloulou et al. 2021) (Fig. 1).
Characterization of hydrophobic ceramic membrane
Morphology
The top surface (i.e., pore geometry), cross-section, and bottom surface can be studied using a scanning electron microscope (SEM). In addition, SEM is able to reveal surface porosity, pore size, and pore size distribution as shown in the micrograph. The results of SEM images of the surface of ceramic membranes modified with silane agents are summarized in Table 2. It is obvious that the modification of the membrane surface using these silane agents has no significant effect on changing the morphological characteristics of the membrane (Fig. 2a–c). Furthermore, previous research works (Chen et al. 2018c; Hubadillah et al. 2018, 2019a) have indicated that the modified membranes had sponge-like structure and creating this structure increase the mechanical strength and reduce transmembrane resistance at the same time. But, Yang et al (2019) displayed that the γ-Y2Si2O7 ceramic membrane had the leaf-like micro and nano-hierarchical structure (Yang et al. 2019).
Liquid entry pressure
The wetting of pores has always been seen as a big problem in the MD process. When salt spreads on the wet membrane, it can lead to create the lower quality of products and this is why important to use a membrane with a high liquid entry pressure (LEP) value (Rácz et al. 2014). In general, LEP is the minimum pressure required for the incoming feed water to penetrate the pores of the dry membrane, so the membrane can no longer function in the MD system (Karanikola et al. 2017). On the other hand, the pore size and membrane hydrophobicity play an important role in LEP because if the pressure of the feed membrane is lower LEP membrane, wetting phenomenon in MD can be avoided (Karanikola et al. 2017). To achieve a high amount of water vapor without any liquid getting inside, the process of membrane distillation needs a special kind of membrane that is superhydrophobic which has a high contact angle and high LEP. This membrane also needs to have evenly spread tiny holes for the vapor to pass through (Shirazi et al. 2012). The special features of MD membranes are that they repel water, very thin, strong, resistant to heat, and have a smooth surface. Besides being water-repellent, a thinner membrane that allows vapor molecules to pass through more easily is a more practical choice (Shirazi et al. 2012). Furthermore, the high LEP of water shows that the membrane is very effective at stopping water from entering the pores. Another crucial factor for improving the amount of water vapor that produced through membrane distillation is the creation of numerous interconnected micropores (Darmanin and Guittard 2015). It is recommended that having a LEP greater than 0.25 MPa (2.5 bar) is a critical way to avoid undesirable wetting caused by pressure and temperature fluctuations during daily operation (Tai et al. 2020).
Furthermore, LEP relies on the range of pore sizes and water resistance of the membrane. High LEP can be obtained by using a membrane which has small pore size (between 0.1 and 0.6 μm) and also, a rough texture and a substantial contact angle are required for this membrane (Zare and Kargari 2018). As mentioned earlier, because of the importance of pore sizes as an effective factor on LEP value and therefore MD processes so it is better to use membranes with pore sizes between 0.1 and 1 μm for membrane distillation experiments (El-Bourawi et al. 2006). As it has been proven, the improvement of flux can be occurred with an increasing the membrane pore size because of change from mass transfer controlled by Knudsen diffusion to mass transfer controlled by Knudsen–viscous transition. Large pores are good for increasing the flux, but they also make it more likely for the pores to get wet (Cath et al. 2004; Alkhudhiri and Hilal 2017). Choosing the right size of pores in a membrane is important to have a good balance between the amount of liquid that passes through the membrane and the energy used. Moreover, the best pore size depends on the characteristics of the liquid being filtered and how the system is being used (Cath et al. 2004; Alkhudhiri and Hilal 2017). It is interesting to note that high LEP in ceramic membranes can achieve in low surface energy, small pore size, high surface roughness, and high surface tension because these aforementioned factors improve the non-wetting properties of membrane surfaces. Based on this, herein, we have summarized the results of previous research works on ceramic membrane distillation and their major characteristics specially LEP in Table 2. As it is obvious, in most of the articles that have used ceramic membrane in the membrane distillation process, the LEP value has been in the range of 0.1 to > 3 bar.
Membrane thickness
The efficiency of the membrane distillation process is influenced by the thickness of the membrane (Eykens et al. 2017). If the membrane was thinner, it helps mass transfer more but also leads to more heat loss and reduces the driving force. Researchers suggest that when desalinating seawater in small-scale experiments is applied, the best thickness for a membrane should be between 10 and 60 µm (Eykens et al. 2017). To improve the ability of a membrane to let substances pass through, fabrication of thin membrane is the best way. However, the thinness should not compromise the strength of the membrane. So, the best thickness of the membrane is thought to be between 30 and 60 µm (Abu-Zeid et al. 2015).
Wettability
The contact angle of the surface is another critical characteristic to identify the water proofness of the ceramic membrane using silane. The results of previous research works conducted on the CMD in the term of changing in the water contact angle after the surface modification are shown in Table 2. According to this table, it can be seen that increasing the contact angle is an important indicator in the success of the silanization process. As mentioned, the use of silane agents can increase the hydrophobicity of the membrane and increase the contact angle of its surface from 120 or 130 to more than 170 degrees. Generally, based on the obtained results we can conclude that hydrophobic modification of ceramic membranes using silane agents significantly changed the hydrophilicity into hydrophobicity for the fabricated membranes.
Pore size analysis
Another important factor impressed by the membrane modification using hydrophobic agents is pore size distribution analysis. Herein, we have discussed the effect of salinization of ceramic membranes on this factor.
The results of previous studies indicated that at the same sintering time, by the surface modification the pore size became smaller but no changes were observed after grafting process and the major reason of that is grafting with silane agents just react with –OH groups on the surface pores as the results of Hubadillah et al (2019a, b) confirmed (Hubadillah et al. 2019a). In another study by Yang et al (2017) conducted on the superhydrophobic modification of ceramic membranes using PFAS for vacuum membrane distillation application, and the results indicated that the pore structure of the modified membrane had little change before and after modification (Yang et al. 2017). The result of hydrophobic surface modification using C16, which was studied by Chen et al (2018a, b, c) confirmed that the pore size distribution had no significant change before and after the silanization process because of the thickness of grafted C16 layer on the inner surface of pore channels was very thin (Chen et al. 2018b). The pore size distribution analysis of alumina ceramic for vacuum membrane distillation which was conducted by Huang et al (2018) showed that before the surface modification of membrane by hydrophobic agent, the average pore size was about 2.4 µm then by the modification process became smaller and reached to 0.4 µm because of partially filling of pores or coated by gels (Huang et al. 2018). However, Dong et al (2019) explained the different results. They showed that the increasing of PDMS content from 30 to 60% had significant influence on the pore size distribution and as a result of that mean pore size of fabricated and modified membranes improved. These outcomes can be used for the simple adjustment of pore structure by the PDMS content in the mixed precursors (Dong et al. 2019).
Some studies have been indicated that the sintering temperature had a significant effect on the pore size of hydrophobic membrane. Chen et al (2018a, b, c) were approved that the characteristics (specially pore size) of ceramic membrane-based alumina hollow fiber are affected by sintering temperature. The results showed that by the increasing sintering temperature from 1300 to 1500 °C, the pore size of membrane become smaller and decreased from 200 to 173 nm. Moreover, they concluded that by increasing the sintering temperature, the particle grain packed of alumina became more densely and as a result of that the pore size characteristics of small pore decreased (Chen et al. 2018c). In another research work, Wang et al (2016) have utilized β-Sialon ceramic hollow fiber membranes for membrane distillation and results demonstrated that sintering temperature had significant effects on the pore size so that as the sintering temperature increased from 1500 to 1650 °C, the pore size of modified membrane using 2 wt% FAS was declined from 1.1 to 0.8 nm (Wang et al. 2016).
The effect of silane agent on the MD performance
The surface of the membrane is reinforced and maintained as a result of chemical bonding and the interactions occurring between molecules. This aspect plays a crucial role in ensuring the membrane’s stability and ability to resist water. However, the ability of immersion methods to make the ceramic membrane hydrophobic can be affected by different factors like the dosage of the silane agent used, the level of hydroxyl groups on the membrane surface, how long the grafting process takes, the number of grafting cycles, and the roughness of the membrane surface (Ahmad et al. 2015).
One of the disadvantages of using these silane agents in the grafting method is the high cost of these materials. Therefore, the optimal concentration of the silane agent must be fully investigated in order to optimize the cost of grafting process (Hubadillah et al. 2019b). In addition, the study on the duration of grafting is also necessary to achieve the maximum reaction between silanol and hydroxyl groups on the surface of the membrane (Hubadillah et al. 2019b).
In the following, an attempt is made to discuss the main parameters in the silane grafting processes, i.e., process duration and the dose/concentration of the silane agent on the ceramic membrane, which are used for membrane distillation.
Silanization time
Various studies have been conducted to investigate the amount of time for the silanization process of ceramic membranes, in which it has been pointed out that the time of the silanization process has a direct relationship with the efficiency of the process (Kujawa et al. 2016; Li et al. 2021). Because in this case, due to the long duration of the process hydrophobicity will make the membrane surface smoother (less surface roughness) and with the increase in time, the contact angle of the membrane surface will increase due to the fact that more concentrations of the silane agent will come into contact with the membrane surface and finally the hydrophobicity will also increase at the same time (Kujawa et al. 2016; Li et al. 2021). Zewdie et al (2022) investigated in detail the effect of silanization time on the ceramic membrane used in the distillation process. In this study, the time of the hydrophobization process varied from 12 to 96 h, and the results showed that the efficiency of the silanization process increased from 3.25 ± 0.1% to 4.6 ± 0.06%, and the best possible state occurred in 72 h. On the other hand, with an excessive increase in time, the degree of the silanization process decreased. This happens because the hydroxyl group on the surface of the ceramic membrane interacts with the Si–O–alkyl groups of the PFAS agent over a long period of time. This interaction helps to decrease the number of hydroxyl groups (Zewdie et al. 2022). In a study conducted by Kujawa et al. (2016), the effect of 4 types of silane agents (C6, C8, C10, and C12) on the hydrophobic properties of ceramic membrane in air gap membrane distillation process under 3-time intervals (first stage: 5 h, the second stage: 15 h, and the third stage: 35 h) was examined. The results of the study indicated that significant changes in the physicochemical properties of the membrane surface and morphology were observed as a result of hydrophobization, so that a noteworthy increase in the water contact angle shows that the surfaces of the ceramic membrane have been effectively modified and their hydrophilic properties become hydrophobic. For example, the contact angle for the C6-modified membrane increased from 110 to 130° with increasing time up to 35 h. But in general, the highest amount of contact angle was obtained for the membranes grafted with C12 (148 degrees flat for 35 h) and with C6 (145 degrees under 35 h) (Kujawa et al. 2016). Zhang et al. (2012) investigated the efficiency of ceramic membrane modified with 2 wt% of C6 silane agent in the membrane distillation process (Table 3) and the results of the study showed that under a total time of 72 h (3 cycles of 24 h) the modification of ceramic membrane surface has improved its hydrophobicity and increased the contact angle of the surface to 136°, which shows that the modification of the membrane surface with the silane agent and the change in its properties have been successfully carried out. The fabricated membrane was also confirmed that it had ability to remove 99–100% of salt in the membrane distillation process (Zhang et al. 2012). Kujawa et al (2013) study evaluated the effect of silanization time on ceramic membranes using C6 and C12 agents. In this study, the investigated time period varied from 1 to 300 h. The results of the study showed that with the increase in contact time, the efficiency of the silanization process also increased, however, the highest increase in efficiency was observed during the first 72 h. On the other hand, by silanizing the surface of the ceramic membrane, the analysis of the contact angle also determined that its hydrophobicity has increased (the contact angle is 136°), which indicates that the process is carried out correctly (Kujawa et al. 2013). But in the study of Kujawa et al. (2016), interesting results were reported. In this study, they tested different types of chemicals such 1H,1H,2H,2H-perfluorooctyltriethoxysilane, n-octyltriethoxysilane, n-octyltrimethoxysilane, and n-octyltrichlorosilane to make a hydrophobic ceramic membrane. They found that using these chemicals, the process took only 5 to 15 min. This short amount of time was enough to change the properties of ceramic membranes from being hydrophilic to hydrophobic. Increasing the time from 5 to 15 min made the contact angle values go up by around 10%. Also, they discovered that the type of silane agent molecules also effects on hydrophobicity. The surface that was modified using perfluoroalkylsilanes had the highest hydrophobicity, with a contact force of 130° after 15 min. However, the membranes treated with alkylsilanes had a lower hydrophobicity, with a contact force of 115° after 15 min. This fact is because the increased size of perfluorinated chains necessitates additional energy for hydration (Table 3) (Kujawa and Kujawski 2016).
Silane concentration
The results of the study by Song et al. (2022) showed that among the different parameters (silane concentration, silanization time, and number of silanization times), it was the concentration of the silane agent that contributed the most to improving the hydrophobic properties of the ceramic membrane used in the distillation process. In this study, the amount of Ghamel silane used ranged from 0. 006 to 0012 mol/liter. The higher the concentration, the greater the contact angle between the substance and the ceramic membrane, causing the membrane to exhibit increased hydrophobicity. Specifically, the angle increased from 131.2 to 156.2°. The explanation is that the solution with a higher concentration contains a larger amount of C2H5O– groups, and these groups readily bond with the hydroxyl groups on the ceramic membrane. This creates a thick layer of a hydrophobic material on the surface of the membrane. So, FAS is successfully attached to Al2O3 ceramic substrate (Song et al. 2022).
Comparative study
In this section, the performance of different types of CMD in terms of flux and salt removal are compared in Table 4. Also, herein, we have tried to discuss the effect of the various effective parameters (temperature, flow rate, input salt concentration) on the membrane distillation process.
Effect of temperature
Temperature is considered as the most important factors affecting the membrane process and so many literatures have reported the effect of feed temperature on permeate vapor flux due to the importance effect of temperature in MD processes (Lee et al. 1997; Zhang et al. 2014). The reason of that is because the performance of membrane distillation process is affected by the increase in exponential pressure with temperature (Lee et al. 1997; Zhang et al. 2014). Due to the temperature disparity on each side of the membrane, the water transforms into vapor and traverses through the membrane and pure water collects or condenses on the other side (Tijing et al. 2015).
The studies found that as the feed temperature is higher, the permeate flux is also higher. This can be explained by the Antoine equation, which shows the connection between the liquid temperature and the vapor pressure balance so that this balance is the main factor that drives the MD process (Qtaishat et al. 2008). The implication of this phenomenon is that a higher permeate flux is obtained at a higher feed temperature due to the corresponding higher vapor pressure. These observations are consistent with the other previous studies (Table 4). According to this table, the operating temperature of MD process varied between 50 and 95 °C, but in general, it can be concluded that the best and highest salt removal efficiency, i.e., > 98%, was obtained in the temperature range between 70 and 90 °C.
The results of a study conducted by Zhang et al. (2012) showed that the fabricated membrane had a high removal rate (99 to 100%) indicating that only water vapor passes through the membrane, but nonvolatile compounds such as NaCl on the feed side enter the membrane. The hydrophobicity of Si3N4 is avoided in the VMD process. When the NaCl solution concentration was fixed at 4 wt%, increasing the temperature of the feed solution from 50 to 70 °C had a positive effect on flux so that the membrane flux increased from 231 to 534 L/m2 day. This is because more water evaporation creates at higher temperature and the steam driving force through the membrane increases. Also, the results approved that the higher concentration of NaCl solution, the lower the permeate flux will be observed. This phenomenon is also observed at 60 and 70 °C, which can be understood by reducing the vapor pressure and increasing the salt concentration, which leads to a decrease in the amount of evaporation on the feed side entering the membranes and a decrease in its flux (Zhang et al. 2012). In another study conducted by Cong et al. (2019) to investigate the efficiency of porous ceramic membrane in membrane distillation process, the permeate flux was between 0.3 and 3.8 kg/m2 h in the temperature range of about 43 to 80 °C for all ceramic tubes. The porosity was variable with the rate of salt removal greater than 99.95%. When the temperature of the feed was increased, the rate of flow showed a rapid increase, holding all other factors constant. This happens because the main reason the MD process works is because of the difference in vapor pressure across the ceramic membrane, and this difference gets bigger as the temperature of the feed increases (Cong et al. 2019). In the study by Chen et al. (2018a, b, c), the operating temperature in membrane distillation ranged from 55 to 75 °C, and the results showed that the water flux of different membranes (M1, M2, M3, and M4) in the VMD process has a positive relationship with temperature. As the temperature increases, an improvement in water flux was observed, which is probably due to the fact that the partial pressure of vapor increases with temperature (Chen et al. 2018b). In a study by Huang et al. (2018), the pure water output flux is 28 L/m2.h when the feed temperature is 50 °C, but when the feed temperature is increased to 70 °C, the vapor pressure is also increased from 12 kPa at 50 °C increased to 31 kPa at 70 °C and a significant increase in flux (37.07 L/m2.h) was observed. Initially, the water possesses a uniform temperature, resulting in accelerated evaporation. The permit rate experiences a minor decline after 30 min of work, as the gradual temperature shift near the membrane surface, resulting from evaporation cooling, takes place (Huang et al. 2018). In the study by Zhang et al. (2014), the operating temperature in the membrane distillation process varied between 50 and 80 °C, and the results showed that the output flux increased rapidly with the increase in feed temperature from 50 to 80 °C for different concentrations of NaCl increased because the driving force of desalination using MD is provided by the gas pressure difference between the two sides of the membrane. Since the pressure on the permeate side is constant at 0.02 bar, the driving force increases with increasing water vapor pressure on the feed side, while the water vapor pressure increases exponentially with increasing temperature. Therefore, higher temperature increases the steam driving force through the membrane. When the concentration of NaCl solution is fixed at 4% by weight, increasing the feed temperature from 50 to 80 °C results in an increase of 446 L/m2 in water infiltration flux (679–233 L/m2 day) (Zhang et al. 2014).
Effect of salt feed concentration
Another operating parameter in evaluating the performance of ceramic membranes used in the membrane process is the amount of salt concentration in the feed entering the membrane.
Studies have shown that the flux in MD with modified ceramic membranes depends on the salt concentration in the feed, so that the water flux values through ceramic membranes which are modified with FAS decrease with an increasing salt concentration in the incoming feed (Krajewski et al. 2006). Based on the obtained results, the increase in salt concentration had significant effect on the increasing NaCl boiling point which can led to decline the water vaporization on surface of membrane (Twibi et al. 2021). However, excesses increase in the salt concentration have negative influence on the membrane permeation because of various reasons, for example, vapor pressure will be reduced as salt concentration increased or it can be contributed due to the concentration polarization and last option which is stated by Twibi et al (2021) (Twibi et al. 2021) is membrane surface fouling. Moreover, this phenomenon can be better explained by the Raoult’s Law in which we can understand that by increasing NaCl concentration the vapor pressure decreased (Zhang et al. 2014).
Alftessi et al. (2022) also used a modified ceramic membrane in the DCMD membrane distillation process. In this study, the input salt concentration ranged from 8 to 40 g/L, and the results showed that with the increase in the input concentration, the output flux of the ceramic membrane decreased from 30 to 12 kg/h per square meter (Alftessi et al. 2022). Also, the results of the study by Larbot and his colleagues in 2004, used a ceramic membrane based on metal oxides of zirconia and aluminum in the membrane distillation process, were consistent with previous results. In this study, the concentration of the salt solution varied from 0.001 to 1 M, and the results showed that at low concentrations, the salt removal rate was between 90 and 96%, while with the increase in concentration to 1 M, the removal efficiency increased to more than 99%, and the concentration its optimum was chosen to be 0.1 M (Bandar et al. 2021).
Effect of flow rate
The effectiveness of ceramic membranes in membrane distillation is contingent upon the input flow rate to the ceramic membrane. According to research, there is a close connection between the movement of liquid and the amount that passes through a material, and this bond is expected to become more pronounced with an increase in the flow rate (Pal and Manna 2010). The main reason for the increase in flux at the same time as the flow rate increases is because of increasing Reynolds number by increasing in the feed flow rate. This phenomena affects the fluid dynamics and increases the heat transfer coefficient, and as result of that reducing the effect of temperature and concentration polarization can be observed (Pal and Manna 2010). Both the mass resistance and the heat transfer resistance decrease at higher Reynolds number as well as the thickness of the boundary layer, which increases the significant driving force for mass transfer through the membrane and thus increases the permeability (Garcıa-Payo et al. 2000; Chen et al. 2020).
Previous studies have also agreed with these reasons. Alftessi et al. (2022) found that the input feed flow and the distillation flux for the prepared ceramic membrane have a direct relationship, so that increasing the feed flow rate from 10 to 20 L per hour caused an increase in the permeate vapor flux (Table 4) (Alftessi et al. 2022). In another study, Donato et al. (2020) evaluated the effect of varying the input flow rate from 65 to 120 L/h and variable temperature from 60 to 70 °C on the performance of the ceramic membrane used in the membrane process. The results of the study showed that by keeping the temperature constant at 60 °C, the output flux from the ceramic membrane increased from 18 to 20 kg/m2 h along with the increase in the flow rate from 65 to 120 L per hour, which is the main reason for heat transfer. It is better inside the flow. Also, under these conditions, the salt removal efficiency was higher than 99% (Donato et al. 2020). In general, it can be concluded that the flow rate has a direct relationship with the amount of flux coming out of the membrane, but it has a lesser effect on the rate of salt absorption, and as mentioned, the amount of flux output is also improved at higher currents.
All in all, based on the obtained results it is obvious that 4 types of membrane processes can have an efficiency in the range of 98–100% depending on the different operating conditions and parameters in salt removal, but on the other hand, the flux of these 4 types of processes is different from each other. DCMD had water flux of 0.13 to 600 per liter in or 1 to 38.2 per kilogram also in VMD process, water flux was 60 to 534 per liter or 20.6 to 44.1 per kilogram.
Future challenges and recommendations
Membrane distillation is regarded as one of the most appealing membrane-based separation technologies, owing to its ability to produce fresh water from high salinity water. As a result, it has garnered noteworthy attention in the field of water treatment especially desalination. This article presents a comprehensive review on the silanization of ceramic membranes intended for employment in membrane distillation processes. However, there are no evidence on the optimization of effective factors on this process. So, we still need to do experiments on full scale plants and watch the different steps closely to solve problems in MD.
New modules and configurations have been added to MD. Although these new systems can help improve MD efficiency, most of them were created in a laboratory. More research is needed to study the materials used and make them more affordable. MD is a helpful method that can be used with other ways to get rid of all liquids and be more sustainable. The complete commercialization of surface-modified ceramic requires the resolution of various hurdles that must be overcome. The fabrication process of ceramic membrane substrate entails a considerably intensive energy expenditure, as its production necessitates exposure to temperatures exceeding 800 °C for prolonged periods. As such, polymer membranes are comparatively less expensive when compared to their counterparts. Furthermore, due to its inherent hydrophilic properties, it is imperative that surface modification be employed prior to the implementation of ceramic membrane as a MD membrane. Incorporating sintering into the manufacturing process poses a challenge in achieving the dual objective of conferring superhydrophobicity or omniphobicity onto membranes concomitantly with proficient control over pore dimensions.
One notable challenge pertaining to the use of ceramic materials is their comparatively high thermal conductivities in relation to those of polymers. This presents as a hindrance toward achieving optimal energy efficiency in the MD process. The development of modification approaches aimed at enhancing thermal efficiency is imperative. MD ceramic membranes that possess distinctive wettability traits typically exhibit inferior flux performance owing to their considerable thickness (> 500 μm), which can be attributed to inherent constraints in the fabrication process, as contrasted with polymeric membranes.
Certain hydrophobic and omniphobic ceramic membranes are subject to structure and performance deterioration over prolonged periods of operation, characterized by fouling, wetting, and reduced flux and rejection. This is attributed to inadequate adhesive forces between fluorination molecules and the membrane surfaces. The occurrence of membrane wetting and fouling in membrane distillation is an undeniable phenomenon. As such, the use of membranes that exhibit high flux recovery following cyclical water flush and chemical cleaning procedures is deemed essential in MD applications. And finally, incorporation of MD, alongside other technologies, in the development of hybrid water treatment systems presents potential for reducing the adverse effects of difficult feed solutions directly on membranes, and possibly decreasing the necessity for specialized membrane surface wettability.
Conclusion
Membrane distillation (MD) is regarded as an inexpensive and auspicious separation method for water desalination owing to its potential to accomplish nearly 100% rejection of dissolved solids. The technology employs a hydrophobic membrane that selectively permits the conduction of vaporized substances while effectively obviating the passage of undesirable solutes in their liquid phase from the feed side. Herein, we discussed the main role of hydrophobization using silane agents. The results confirm that silanization of CM in the MD processes has no significant effect on the surface morphology of the fabricated membrane, while it is found that the other characterizations of ceramic membrane (hydrophobicity, porosity, thickness, wettability, pore size) is influenced by silanization. This review indicated that the optimal properties of MD using CM was porosity (30%–90%), LEP (> 0.5 bar), thickness (20–200 µm), high water contact angel (> 1300), and pore size (0.1–1.0 µm) to ensure high flux and rejection performance. All in all, this review suggests a critical points of using ceramic membrane used for MD process with focusing on optimization of main operational parameters that can be useful for applying in future of this method in full scale operation.
Data availability
Not applicable.
Abbreviations
- VMD:
-
Vacuum membrane distillation
- SGMD:
-
Sweeping gas membrane distillation
- AGMD:
-
Air gap membrane distillation
- DCMD:
-
Direct contact membrane distillation
- MD:
-
Membrane distillation
- PFAS:
-
Perfluoroalkylsilane
- FAS:
-
Fluoroalkylsilanes
- PFDT:
-
1H,1H,2H,2H-perfluorodecanethiol
- APTES:
-
3-Aminopropyltriethoxysilane
- WCA:
-
Water contact angel
- TEOS:
-
Tetraethoxysilane
- C16:
-
Hexadecyltrimethoxysilane
- MTS:
-
Methyltrichlorosilane
- PFDA:
-
1H,1H,2H,2H-perfluorodecyl acrylate
- PFOTES (C6):
-
1H,1H,2H,2H-perfluorooctyltriethoxysilane
- PFTES (C8):
-
1H,1H,2H,2H-perfluorodecyltriethoxysilane
- C12:
-
1H,1H,2H,2H-perfluorotetradecyltriethoxysilane
- PDTS:
-
1H,1H,2H,2H-perfluorodecytriethoxysilane
- FDTS:
-
1H,1H,2H,2H-perfluorodecyltrichlorosilane
- FAS17:
-
1H, 1H, 2H, 2H-perfluorodecyltrimethoxysilane
- FTCS:
-
1H, 1H, 2H, 2H-perfluorododecyltrichlorosilane
- PPFDA:
-
1H,1H,2H,2H-perfluorodecyl acrylate
- C6OMe3:
-
N-Octyltrimethoxysilane
- C6Cl3:
-
N-Octyltrichlorosilane
- C6OEt3:
-
N-Octyltriethoxysilane
- RO:
-
Reverse osmosis
- LEP:
-
Liquid entry pressure
- SEM:
-
Scanning electron microscope
References
Abu-Zeid MAE-R, Zhang Y, Dong H, Zhang L, Chen H-L, Hou L (2015) A comprehensive review of vacuum membrane distillation technique. Desalination 356:1–14
Ahmad N, Leo C, Ahmad A, Ramli W (2015) Membranes with great hydrophobicity: a review on preparation and characterization. Sep Purif Rev 44:109–134
Alftessi SA, Othman MHD, Adam MRB, Farag TM, Tai ZS, Raji YO, Rahman MA, Jaafar J, Ismail AF, Bakar SA (2022) Hydrophobic silica sand ceramic hollow fiber membrane for desalination via direct contact membrane distillation. Alex Eng J 61:9609–9621
Alkhudhiri A, Hilal N (2017) Air gap membrane distillation: a detailed study of high saline solution. Desalination 403:179–186
Aloulou H, Aloulou W, Daramola MO, Amar RB (2021) Silane-grafted sand membrane for the treatment of oily wastewater via air gap membrane distillation: study of the efficiency in comparison with microfiltration and ultrafiltration ceramic membranes. Mater Chem Phys 261:124186
Bandar KB, Alsubei MD, Aljlil SA, Darwish NB, Hilal N (2021) Membrane distillation process application using a novel ceramic membrane for Brackish water desalination. Desalination 500:114906
Bhushan B, Her EK (2010) Fabrication of superhydrophobic surfaces with high and low adhesion inspired from rose petal. Langmuir 26:8207–8217
Budel B (1963) Silicone rubber vapor diffusionin saline water distillation
Cath TY, Adams VD, Childress AE (2004) Experimental study of desalination using direct contact membrane distillation: a new approach to flux enhancement. J Membr Sci 228:5–16
Cerneaux S, Strużyńska I, Kujawski WM, Persin M, Larbot A (2009) Comparison of various membrane distillation methods for desalination using hydrophobic ceramic membranes. J Membr Sci 337:55–60
Chen L-H, Chen Y-R, Huang A, Chen C-H, Su D-Y, Hsu C-C, Tsai F-Y, Tung K-L (2018a) Nanostructure depositions on alumina hollow fiber membranes for enhanced wetting resistance during membrane distillation. J Membr Sci 564:227–236
Chen X, Gao X, Fu K, Qiu M, Xiong F, Ding D, Cui Z, Wang Z, Fan Y, Drioli E (2018b) Tubular hydrophobic ceramic membrane with asymmetric structure for water desalination via vacuum membrane distillation process. Desalination 443:212–220
Chen Y-R, Chen L-H, Chen C-H, Ko C-C, Huang A, Li C-L, Chuang C-J, Tung K-L (2018c) Hydrophobic alumina hollow fiber membranes for sucrose concentration by vacuum membrane distillation. J Membr Sci 555:250–257
Chen L, Xu P, Wang H (2020) Interplay of the factors affecting water flux and salt rejection in membrane distillation: a state-of-the-art critical review. Water 12:2841
Cong S, Liu X, Guo F (2019) Membrane distillation using surface modified multi-layer porous ceramics. Int J Heat Mass Transf 129:764–772
Darmanin T, Guittard F (2015) Superhydrophobic and superoleophobic properties in nature. Mater Today 18:273–285
Das R, Sondhi K, Majumdar S, Sarkar S (2016) Development of hydrophobic clay–alumina based capillary membrane for desalination of brine by membrane distillation. Asian Ceram Soc 4:243–251
Ding Z, Liu L, Li Z, Ma R, Yang Z (2006) Experimental study of ammonia removal from water by membrane distillation (MD): the comparison of three configurations. J Membr Sci 286:93–103
Donato L, Garofalo A, Drioli E, Alharbi O, Aljlil S, Criscuoli A, Algieri C (2020) Improved performance of vacuum membrane distillation in desalination with zeolite membranes. Sep Purif Technol 237:116376
Dong B-B, Wang F-H, Yang M-Y, Yu J-L, Hao L-Y, Xu X, Wang G, Agathopoulos S (2019) Polymer-derived porous SiOC ceramic membranes for efficient oil-water separation and membrane distillation. J Membr Sci 579:111–119
El-Bourawi M, Ding Z, Ma R, Khayet M (2006) A framework for better understanding membrane distillation separation process. J Membr Sci 285:4–29
Elimelech M, Phillip WA (2011) The future of seawater desalination: energy, technology, and the environment. Science 333:712–717
Eykens L, De Sitter K, Dotremont C, Pinoy L, Van der Bruggen B (2017) Membrane synthesis for membrane distillation: a review. Sep Purif Technol 182:36–51
Fan Y, Chen S, Zhao H, Liu Y (2017) Distillation membrane constructed by TiO2 nanofiber followed by fluorination for excellent water desalination performance. Desalination 405:51–58
Fang H, Gao J, Wang H, Chen C (2012) Hydrophobic porous alumina hollow fiber for water desalination via membrane distillation process. J Membr Sci 403:41–46
García-Fernández L, Wang B, García-Payo M, Li K, Khayet M (2017) Morphological design of alumina hollow fiber membranes for desalination by air gap membrane distillation. Desalination 420:226–240
Garcıa-Payo M, Izquierdo-Gil MA, Fernández-Pineda C (2000) Air gap membrane distillation of aqueous alcohol solutions. J Membr Sci 169:61–80
Gleick PH (1993) Water in crisis. Oxford University Press, New York
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P (2009) Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res 43:2317–2348
Hendren Z, Brant J, Wiesner M (2009) Surface modification of nanostructured ceramic membranes for direct contact membrane distillation. J Membr Sci 331:1–10
Huang C-Y, Ko C-C, Chen L-H, Huang C-T, Tung K-L, Liao Y-C (2018) A simple coating method to prepare superhydrophobic layers on ceramic alumina for vacuum membrane distillation. Sep Purif Technol 198:79–86
Hubadillah SK, Othman MHD, Matsuura T, Rahman MA, Jaafar J, Ismail A, Amin SZM (2018) Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep Purif Technol 205:22–31
Hubadillah SK, Othman MHD, Ismail A, Rahman MA, Jaafar J (2019a) A low cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep Purif Technol 214:31–39
Hubadillah SK, Tai ZS, Othman MHD, Harun Z, Jamalludin MR, Rahman MA, Jaafar J, Ismail AF (2019b) Hydrophobic ceramic membrane for membrane distillation: a mini review on preparation, characterization, and applications. Sep Purif Technol 217:71–84
Karanikola V, Corral AF, Jiang H, Sáez AE, Ela WP, Arnold RG (2017) Effects of membrane structure and operational variables on membrane distillation performance. J Membr Sci 524:87–96
Khemakhem S, Amar RB (2011) Grafting of fluoroalkylsilanes on microfiltration Tunisian clay membrane. Ceram Int 37:3323–3328
Khemakhem S (2022) Preparation and evaluation of hydrophobic grafted ceramic membrane: for application in water desalination. Wastewater Treat 179
Ko C-C, Chen C-H, Chen Y-R, Wu Y-H, Lu S-C, Hu F-C, Li C-L, Tung K-L (2017) Increasing the performance of vacuum membrane distillation using micro-structured hydrophobic aluminum hollow fiber membranes. Appl Sci 7:357
Ko C-C, Ali A, Drioli E, Tung K-L, Chen C-H, Chen Y-R, Macedonio F (2018) Performance of ceramic membrane in vacuum membrane distillation and in vacuum membrane crystallization. Desalination 440:48–58
Krajewski SR, Kujawski W, Bukowska M, Picard C, Larbot A (2006) Application of fluoroalkylsilanes (FAS) grafted ceramic membranes in membrane distillation process of NaCl solutions. J Membr Sci 281:253–259
Kujawa J, Kujawski W (2016) Functionalization of ceramic metal oxide powders and ceramic membranes by perfluoroalkylsilanes and alkylsilanes possessing different reactive groups: Physicochemical and tribological properties. ACS Appl Mater Interfaces 8:7509–7521
Kujawa J, Kujawski W, Koter S, Rozicka A, Cerneaux S, Persin M, Larbot A (2013) Efficiency of grafting of Al2O3, TiO2 and ZrO2 powders by perfluoroalkylsilanes. Colloids Surf A Physicochem Eng 420:64–73
Kujawa J, Cerneaux S, Koter S, Kujawski W (2014) Highly efficient hydrophobic titania ceramic membranes for water desalination. ACS Appl Mater Interfaces 6:14223–14230
Kujawa J, Cerneaux S, Kujawski W, Bryjak M, Kujawski J (2016) How to functionalize ceramics by perfluoroalkylsilanes for membrane separation process? Properties and application of hydrophobized ceramic membranes. ACS Appl Mater Interfaces 8:7564–7577
Kujawa J, Cerneaux S, Kujawski W, Knozowska K (2017) Hydrophobic ceramic membranes for water desalination. Appl Sci 7:402
Lanjewar T, Satyakam A, Varma MN (2022) Low-cost hydrophobic cenosphere ceramic membrane for the desalination application using direct contact membrane distillation. Arab J Sci Eng 47:1–16
Lattemann S, Höpner T (2008) Environmental impact and impact assessment of seawater desalination. Desalination 220:1–15
Lee Y, Jeong J, Youn I, Lee W (1997) Modified liquid displacement method for determination of pore size distribution in porous membranes. J Membr Sci 130:149–156
Lee J, Ha J-H, Song I-H (2016) Improving the antifouling properties of ceramic membranes via chemical grafting of organosilanes. Sep Sci Technol 51:2420–2428
Li L, Wang J-W, Zhong H, Hao L-Y, Abadikhah H, Xu X, Chen C-S, Agathopoulos S (2017) Novel α-Si3N4 planar nanowire superhydrophobic membrane prepared through in-situ nitridation of silicon for membrane distillation. J Membr Sci 543:98–105
Li L, Abadikhah H, Wang J-W, Xu X, Agathopoulos S (2018) One-step synthesis of flower-like Si2N2O nanowires on the surface of porous SiO2 ceramic membranes for membrane distillation. Mater Lett 232:74–77
Li B, Yun Y, Wang M, Li C, Yang W, Li J, Liu G (2021) Superhydrophobic polymer membrane coated by mineralized β-FeOOH nanorods for direct contact membrane distillation. Desalination 500:114889
Mishra BK, Kumar P, Saraswat C, Chakraborty S, Gautam A (2021) Water security in a changing environment: concept, challenges and solutions. Water 13:490
Mizoshita N, Goto Y, Inagaki S, Shimada T (2011) Organosilane compound and organosilica obtained therefrom, Google patents
Omar NMA, Othman MHD, Tai ZS, Kurniawan TA, El-badawy T, Goh PS, Othman NH, Rahman MA, Jaafar J, Ismail AF (2022) Bottlenecks and recent improvement strategies of ceramic membranes in membrane distillation applications: a review. J Eur Ceram Soc 42:5179–5194
Pagliero M, Bottino A, Comite A, Costa C (2020) Silanization of tubular ceramic membranes for application in membrane distillation. J Membr Sci 601:117911
Pal P, Manna AK (2010) Removal of arsenic from contaminated groundwater by solar-driven membrane distillation using three different commercial membranes. Water Res 44:5750–5760
Parani S, Oluwafemi OS (2021) Membrane distillation: recent configurations, membrane surface engineering, and applications. Membranes 11:934
Picard C, Larbot A, Sarrazin J, Janknecht P, Wilderer P (2001) Ceramic membranes for ozonation in wastewater treatment, annales de chimie science des matériaux. Elsevier, Amsterdam, pp 13–22
Qtaishat M, Matsuura T, Kruczek B, Khayet M (2008) Heat and mass transfer analysis in direct contact membrane distillation. Desalination 219:272–292
Rácz G, Kerker S, Kovács Z, Vatai G, Ebrahimi M, Czermak P (2014) Theoretical and experimental approaches of liquid entry pressure determination in membrane distillation processes. Period Polytech Chem Eng 58:81–91
Ren C, Fang H, Gu J, Winnubst L, Chen C (2015) Preparation and characterization of hydrophobic alumina planar membranes for water desalination. J Eur Ceram Soc 35:723–730
Ren L-F, Xia F, Chen V, Shao J, Chen R, He Y (2017) TiO2-FTCS modified superhydrophobic PVDF electrospun nanofibrous membrane for desalination by direct contact membrane distillation. Desalination 423:1–11
Shirazi MMA, Kargari A, Shirazi MJA (2012) Direct contact membrane distillation for seawater desalination. Desalin Water Treat 49:368–375
Shirazi A, Mohammad M, Kargari A (2015) A review on applications of membrane distillation (MD) process for wastewater treatment. J Membr Sci Res 1:101–112
Song Y, Chen Z, Chen J-H (2022) Preparation and characterisation of the novel hydrophobic FAS/α-Al2O3 composite membrane for membrane distillation. Mater Res Innov 26:168–175
Summers EK, Arafat HA (2012) Energy efficiency comparison of single-stage membrane distillation (MD) desalination cycles in different configurations. Desalination 290:54–66
Tai ZS, Abd Aziz MH, Othman MHD, Mohamed Dzahir MIH, Hashim NA, Koo KN, Hubadillah SK, Ismail AF, Rahman AM, Jaafar J (2020) Ceramic membrane distillation for desalination. Sep Purif Rev 49:317–356
Tijing LD, Woo YC, Choi J-S, Lee S, Kim S-H, Shon HK (2015) Fouling and its control in membrane distillation—a review. J Membr Sci 475:215–244
Twibi MF, Othman MHD, Hubadillah SK, Alftessi SA, Adam MRB, Ismail AF, Rahman MA, Jaafar J, Raji YO, Abd Aziz MH (2021) Hydrophobic mullite ceramic hollow fibre membrane (Hy-MHFM) for seawater desalination via direct contact membrane distillation (DCMD). J Eur Ceram Soc 41:6578–6585
Uddin MG, Nash S, Olbert AI (2021) A review of water quality index models and their use for assessing surface water quality. Ecol Indic 122:107218
Van Vliet MT, Jones ER, Flörke M, Franssen WH, Hanasaki N, Wada Y, Yearsley JR (2021) Global water scarcity including surface water quality and expansions of clean water technologies. Environ Res Lett 16:024020
Wang J-W, Li L, Zhang J-W, Xu X, Chen C-S (2016) β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J Eur Ceram Soc 36:59–65
Wang T, Yun Y, Wang M, Li C, Liu G, Yang W (2019) Superhydrophobic ceramic hollow fiber membrane planted by ZnO nanorod-array for high-salinity water desalination. J Taiwan Inst Chem Eng 105:17–27
Wang F, Dong B, Ke N, Yang M, Qian R, Wang J, Yu J, Hao L, Yin L, Xu X (2021) Superhydrophobic β-Sialon-mullite ceramic membranes with high performance in water treatment. Ceram Int 47:8375–8381
Yang Y, Liu Q, Wang H, Ding F, Jin G, Li C, Meng H (2017) Superhydrophobic modification of ceramic membranes for vacuum membrane distillation. Chin J Chem Eng 25:1395–1401
Yang M-Y, Wang J-W, Li L, Dong B-B, Xin X, Agathopoulos S (2019) Fabrication of low thermal conductivity yttrium silicate ceramic flat membrane for membrane distillation. J Eur Ceram Soc 39:442–448
Yi H, Han Y, Zhuo Y (2013) Effect of combined pretreatment of waste activated sludge for anaerobic digestion process. Procedia Environ Sci 18:716–721
Yun C-Y, Kim W-Y, Son D-J, Kim D-G, Chang D, Chang S-O, Sunwoo Y, Hong K-H (2015) Fabrication of tubular-type MF ceramic membrane with enhanced permeability by addition of PMMA in the support and evaluation of physical characteristics for wastewater treatment. Ceram Int 41:10788–10794
Zare S, Kargari A (2018) Membrane properties in membrane distillation, emerging technologies for sustainable desalination handbook. Elsevier, Amsterdam, pp 107–156
Zewdie TM, Habtu NG, Dutta A, Van der Bruggen B (2022) Flat sheet metakaolin ceramic membrane for water desalination via direct contact membrane distillation. J Water Reuse Desalin 12:131–156
Zhang J-W, Fang H, Hao L-Y, Xu X, Chen C-S (2012) Preparation of silicon nitride hollow fibre membrane for desalination. Mater Lett 68:457–459
Zhang J-W, Fang H, Wang J-W, Hao L-Y, Xu X, Chen C-S (2014) Preparation and characterization of silicon nitride hollow fiber membranes for seawater desalination. J Membr Sci 450:197–206
Zhang Z, Yang J, Qi R, Huang J, Chen H, Zhang H (2023) Development of hydrophobic coal-fly-ash-based ceramic membrane for vacuum membrane distillation. Materials 16:3153
Zhou W, Zhang L, Wu P, Liu Y, Cai Y, Zhao X (2020) An effective method for improving the permeation flux of a ceramic membrane: single-matrix spherical ceramic membrane. J Hazard Mater 400:123183
Acknowledgements
The authors hereby express their gratitude to the Student Research Committee of Kermanshah University of Medical Sciences for financial support (Grant No: 4020795, Ethical Code: IR.KUMS.REC.1402.404). Besides, this work was a initial part of the requirements for Ph.D thesis (Ethical Code:IR.KUMS.REC.1402.502) of Danial Nayeri in Faculty of Health, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Consent to participate
Not applicable.
Consent form publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nayeri, D., Mousavi, S.A. A critical review on the effect of silanization on the ceramic membrane distillation (CMD): performance, operational factors, and characterization. Appl Water Sci 14, 114 (2024). https://doi.org/10.1007/s13201-024-02178-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02178-3