Abstract
Alpha-particle radionuclide-antibody conjugates are being clinically evaluated against solid tumors even when they moderately express the targeted markers. At this limit of lower tumor-absorbed doses, to maintain efficacy, the few(er) intratumorally delivered alpha-particles need to traverse/hit as many different cancer cells as possible. We complement antibody-radioconjugate therapies with a separate nanocarrier delivering a fraction of the same total injected radioactivity to tumor regions geographically different than those affected by targeting antibodies; these carrier-cocktails collectively distribute the alpha-particle emitters better.
Methods
The efficacy of actinium-225 delivered by our carrier-cocktails was assessed in vitro and on mice with orthotopic MDA-MB-436 and/or MDA-MB-231 triple-negative breast cancers and/or an ectopic BxPC3 pancreatic cancer. Cells/tumors were chosen to express low-to-moderate levels of HER1, as model antibody-targeted marker.
Results
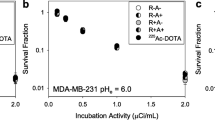
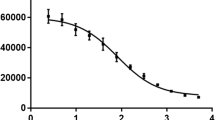
Independent of cell line, antibody-radioconjugates were most lethal on cell monolayers. On spheroids, with radii greater than alpha-particles’ range, carrier-cocktails improved killing efficacy (p < 0.0500). Treatment with carrier-cocktails decreased the MDA-MB-436 and MDA-MB-231 orthotopic tumor volumes by 73.7% and 72.1%, respectively, relative to treatment with antibody-radioconjugates alone, at same total injected radioactivity; these carrier-cocktails completely eliminated formation of spontaneous metastases vs. 50% and 25% elimination in mice treated with antibody-radioconjugates alone. In BxPC3 tumor-bearing mice, carrier-cocktails increased the median survival to 25–26 days (in male–female animals) vs. 20–21 days of mice treated with antibody-radioconjugates alone (vs. 17 days for non-treated animals). Survival with carrier-cocktail radiotherapy was further prolonged by pre-injecting low-dose, standard-of-care, gemcitabine (p = 0.0390).
Conclusion
Tumor-agnostic carrier-cocktails significantly enhance the therapeutic efficacy of existing alpha-particle radionuclide-antibody treatments.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jang A, Kendi AT, Johnson GB, Halfdanarson TR, Sartor O. Targeted alpha-particle therapy: a review of current trials. Int J Mol Sci. 2023;24. https://doi.org/10.3390/ijms241411626.
Karlsson J. Abstract ND07: HER2-TTC: a targeted thorium conjugate to treat HER2 expressing cancers with potent alpha radiation. Cancer Research. 2021;81:ND07-ND. https://doi.org/10.1158/1538-7445.Am2021-nd07.
Yard BD, Gopal P, Bannik K, Siemeister G, Hagemann UB, Abazeed ME. Cellular and genetic determinants of the sensitivity of cancer to α-particle irradiation. Can Res. 2019;79:5640–51. https://doi.org/10.1158/0008-5472.can-19-0859.
Howe A, Bhatavdekar O, Salerno D, Josefsson A, Pacheco-Torres J, Bhujwalla ZM, et al. Combination of carriers, with complementary intratumoral microdistributions of delivered α-particles, may realize the promise for actinium-225 in large solid tumors. J Nucl Med. 2022;63:1223–30. https://doi.org/10.2967/jnumed.121.262992.
Katugampola S, Wang J, Prasad A, Sofou S, Howell RW. Predicting response of micrometastases with MIRDcell V3: proof of principle with 225Ac-DOTA encapsulating liposomes that produce different activity distributions in tumor spheroids. Eur J Nucl Med Mol Imaging. 2022;49:3989–99. https://doi.org/10.1007/s00259-022-05878-7.
Kratochwil C, Haberkorn U, Giesel FL. 225Ac-PSMA-617 for therapy of prostate cancer. Semin Nucl Med. 2020;50:133–40. https://doi.org/10.1053/j.semnuclmed.2020.02.004.
Graff CP, Wittrup KD. Theoretical analysis of antibody targeting of tumor spheroids: importance of dosage for penetration and affinity for retention. Can Res. 2003;63:1288–96.
Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–28. https://doi.org/10.2967/jnumed.108.058651.
Nair R, Prasad A, Bhatavdekar O, Sarkar A, Gabrielson KL, Sofou S. Combined, yet separate: cocktails of carriers (not drugs) for α-particle therapy of solid tumors expressing moderate-to-low levels of targetable markers. bioRxiv. 2023:2023.07.31.551152. https://doi.org/10.1101/2023.07.31.551152.
Prasad A, Nair R, Bhatavdekar O, Howe A, Salerno D, Sempkowski M, et al. Transport-driven engineering of liposomes for delivery of α-particle radiotherapy to solid tumors: effect on inhibition of tumor progression and onset delay of spontaneous metastases. Eur J Nucl Med Mol Imaging. 2021;48:4246–58. https://doi.org/10.1007/s00259-021-05406-z.
Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60:1421–34.
Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, et al. The HER-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8:307–25. https://doi.org/10.1634/theoncologist.8-4-307.
Salerno D, Howe A, Bhatavdekar O, Josefsson A, Pacheco-Torres J, Bhujwalla ZM, et al. Two diverse carriers are better than one: a case study in α-particle therapy for prostate specific membrane antigen-expressing prostate cancers. Bioeng Transl Med. 2022;7:e10266. https://doi.org/10.1002/btm2.10266.
McDevitt MR, Ma D, Simon J, Frank RK, Kiefer GE, Scheinberg DA. Design and synthesis of actinium-225 radioimmunopharmaceuticals. Appl Radiat Isot. 2002;57:841–7.
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. https://doi.org/10.1038/nprot.2006.339.
Song H, Hobbs RF, Vajravelu R, Huso DL, Esaias C, Apostolidis C, et al. Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res. 2009;69:8941–8. https://doi.org/10.1158/0008-5472.CAN-09-1828.
Kondo M, Cai Z, Chan C, Forkan N, Reilly RM. [225Ac]Ac- and [111In]In-DOTA-trastuzumab theranostic pair: cellular dosimetry and cytotoxicity in vitro and tumour and normal tissue uptake in vivo in NRG mice with HER2-positive human breast cancer xenografts. EJNMMI Radiopharmacy Chem. 2023;8:24. https://doi.org/10.1186/s41181-023-00208-0.
Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–28. https://doi.org/10.1002/stem.160413.
Stras S, Howe A, Prasad A, Salerno D, Bhatavdekar O, Sofou S. Growth of metastatic triple-negative breast cancer is inhibited by deep tumor-penetrating and slow tumor-clearing chemotherapy: the case of tumor-adhering liposomes with interstitial drug release. Mol Pharm. 2020;17:118–31. https://doi.org/10.1021/acs.molpharmaceut.9b00812.
Zhu C, Sempkowski M, Holleran T, Linz T, Bertalan T, Josefsson A, et al. Alpha-particle radiotherapy: for large solid tumors diffusion trumps targeting. Biomaterials. 2017;130:67–75. https://doi.org/10.1016/j.biomaterials.2017.03.035.
Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers. 2017;9:157.
Akudugu JM, Howell RW. A method to predict response of cell populations to cocktails of chemotherapeutics and radiopharmaceuticals: validation with daunomycin, doxorubicin, and the alpha particle emitter (210)Po. Nucl Med Biol. 2012;39:954–61. https://doi.org/10.1016/j.nucmedbio.2012.01.011.
Milenic DE, Baidoo KE, Kim Y-S, Brechbiel MW. Evaluation of cetuximab as a candidate for targeted α-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. MAbs. 2015;7:255–64. https://doi.org/10.4161/19420862.2014.985160.
Duan L, Yang L, Jin J, Yang F, Liu D, Hu K, et al. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics. 2020;10:462–83. https://doi.org/10.7150/thno.37593.
Alaouie A, Sofou S. Liposomes with triggered content release for cancer therapy. J Biomed Nanotechnology. 2008;4:234–44.
Deal KA, Davis IA, Mirzadeh S, Kennel SJ, Brechbiel MW. Improved in vivo stability of actinium-225 macrocyclic complexes. J Med Chem. 1999;42:2988–92. https://doi.org/10.1021/jm990141f.
Song H, Hobbs RF, Vajravelu R, Huso DL, Esaias C, Apostolidis C, et al. Radioimmunotherapy of breast cancer metastases with α-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Can Res. 2009;69:8941–8. https://doi.org/10.1158/0008-5472.Can-09-1828.
Jaggi JS, Kappel BJ, McDevitt MR, Sgouros G, Flombaum CD, Cabassa C, et al. Efforts to control the errant products of a targeted in vivo generator. Cancer Res. 2005;65:4888–95.
Jurcic JG. Targeted alpha-particle therapy for hematologic malignancies. Semin Nucl Med. 2020;50:152–61. https://doi.org/10.1053/j.semnuclmed.2019.09.002.
Acknowledgements
The authors thank Dr. George Sgouros at Johns Hopkins University for help with the dosimetry calculations, and Ms. Pooja Hariharan and Mr. Rohit Chaudhari for assistance with animal handling.
Funding
This work was partially supported by grants from the W.W. Smith Charitable Trust, the Allegheny Health Network-Johns Hopkins Cancer Research Fund, and the Elsa U. Pardee Foundation.
Author information
Authors and Affiliations
Contributions
Material preparation and data collection were performed by Rajiv Nair, Aprameya Prasad, Omkar Bhatavdekar, and Aira Sarkar. Analysis was performed by Rajiv Nair, Aprameya Prasad, Kathleen Gabrielson, and Stavroula Sofou. The first draft of the manuscript was written by Stavroula Sofou, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, R.R., Prasad, A., Bhatavdekar, O. et al. Combined, yet separate: cocktails of carriers (not drugs) for actinium-225 α-particle therapy of solid tumors expressing moderate-to-low levels of targetable markers. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06710-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06710-0