当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Process Intensification of the Continuous Synthesis of Bio-Derived Monomers for Sustainable Coatings Using a Taylor Vortex Flow Reactor
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2024-05-09 , DOI: 10.1021/acs.oprd.3c00462 Matthew D. Edwards 1 , Matthew T. Pratley 1 , Charles M. Gordon 2 , Rodolfo I. Teixeira 1 , Hamza Ali 1 , Irfhan Mahmood 1 , Reece Lester 1 , Ashley Love 1 , Johannes G. H. Hermens 3 , Thomas Freese 3 , Ben L. Feringa 3 , Martyn Poliakoff 1 , Michael W. George 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2024-05-09 , DOI: 10.1021/acs.oprd.3c00462 Matthew D. Edwards 1 , Matthew T. Pratley 1 , Charles M. Gordon 2 , Rodolfo I. Teixeira 1 , Hamza Ali 1 , Irfhan Mahmood 1 , Reece Lester 1 , Ashley Love 1 , Johannes G. H. Hermens 3 , Thomas Freese 3 , Ben L. Feringa 3 , Martyn Poliakoff 1 , Michael W. George 1
Affiliation
|
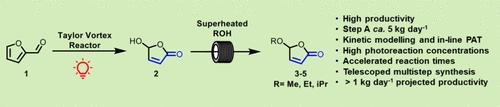
We describe the optimization and scale-up of two consecutive reaction steps in the synthesis of bio-derived alkoxybutenolide monomers that have been reported as potential replacements for acrylate-based coatings ( Sci. Adv. 2020, 6, eabe0026 ). These monomers are synthesized by (i) oxidation of furfural with photogenerated singlet oxygen followed by (ii) thermal condensation of the desired 5-hydroxyfuranone intermediate product with an alcohol, a step which until now has involved a lengthy batch reaction. The two steps have been successfully telescoped into a single kilogram-scale process without any need to isolate the 5-hydroxyfuranone between the steps. Our process development involved FTIR reaction monitoring, FTIR data analysis via 2D visualization, and two different photoreactors: (i) a semicontinuous photoreactor based on a modified rotary evaporator, where FTIR and 2D correlation spectroscopy (2D-COS) revealed the loss of the methyl formate coproduct, and (ii) our fully continuous Taylor Vortex photoreactor, which enhanced the mass transfer and permitted the use of near-stoichiometric equivalents of O2. The use of in-line FTIR monitoring and modeling greatly accelerated process optimization in the Vortex reactor. This led to scale-up of the photo-oxidation in 85% yield with a projected productivity of 1.3 kg day–1 and a space-time yield of 0.06 mol day–1 mL–1. Higher productivities could be achieved while sacrificing yield (e.g., 4 kg day–1 at 40% yield). The use of superheated methanol at 200 °C in a pressurized thermal flow reactor accelerated the second step, the thermal condensation of 5-hydroxyfuranone, from a 20 h batch reflux reaction (0.5 L, 85 g) to a space time of <1 min in a reactor only 3 mL in volume operating with projected productivities of >700 g day–1. Proof of concept for telescoping the two steps was established with an overall two-step yield of 67%, producing a process with a projected productivity of 1.1 kg day–1 for the methoxybutenolide monomer without any purification of the 5-hydroxyfuranone intermediate.
中文翻译:

使用泰勒涡流反应器连续合成可持续涂料的生物衍生单体的工艺强化
我们描述了生物源烷氧基丁烯内酯单体合成中两个连续反应步骤的优化和放大,这些单体已被报道为丙烯酸酯基涂料的潜在替代品(Sci. Adv. 2020 , 6 ,eabe0026 )。这些单体的合成方法如下:(i) 用光生单线态氧氧化糠醛,然后 (ii) 将所需的 5-羟基呋喃酮中间产物与醇热缩合,这一步骤迄今为止涉及漫长的间歇反应。这两个步骤已成功地合并为一个公斤级的工艺,无需在步骤之间分离 5-羟基呋喃酮。我们的工艺开发涉及 FTIR 反应监测、通过2D 可视化进行 FTIR 数据分析以及两种不同的光反应器:(i) 基于改进的旋转蒸发器的半连续光反应器,其中 FTIR 和 2D 相关光谱 (2D-COS) 显示甲基的损失(ii) 我们的完全连续泰勒涡旋光反应器,它增强了传质并允许使用接近化学计量当量的 O 2。在线 FTIR 监测和建模的使用极大地加速了涡流反应器的工艺优化。这使得光氧化的产率扩大到 85%,预计生产率为 1.3 kg day –1,时空产率为 0.06 mol day –1 mL –1。可以在牺牲产量的同时实现更高的生产率(例如,4 kg day –1,产量为 40%)。在加压热流反应器中使用 200 °C 的过热甲醇加速了第二步,即 5-羟基呋喃酮的热缩合,从 20 小时的间歇回流反应(0.5 L,85 g)缩短至 <1 分钟的空间时间在体积仅为 3 mL 的反应器中运行,预计生产率 >700 g day –1。建立了伸缩两个步骤的概念验证,两步总产率为 67%,生产出甲氧基丁烯酸内酯单体的预计生产率为 1.1 kg day -1的工艺,无需对 5-羟基呋喃酮中间体进行任何纯化。
更新日期:2024-05-09
中文翻译:

使用泰勒涡流反应器连续合成可持续涂料的生物衍生单体的工艺强化
我们描述了生物源烷氧基丁烯内酯单体合成中两个连续反应步骤的优化和放大,这些单体已被报道为丙烯酸酯基涂料的潜在替代品(Sci. Adv. 2020 , 6 ,































 京公网安备 11010802027423号
京公网安备 11010802027423号