当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anaerobic cyanides oxidation with bimetallic modulation of biological toxicity and activity for nitrite reduction
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2024-05-07 , DOI: 10.1016/j.jhazmat.2024.134540 Acong Chen , Haoling Li , Haizhen Wu , Zhaohui Song , Yao Chen , Heng Zhang , Zijun Pang , Zhi Qin , Yulun Wu , Xianghong Guan , Hua Huang , Zemin Li , Guanglei Qiu , Chaohai Wei
Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2024-05-07 , DOI: 10.1016/j.jhazmat.2024.134540 Acong Chen , Haoling Li , Haizhen Wu , Zhaohui Song , Yao Chen , Heng Zhang , Zijun Pang , Zhi Qin , Yulun Wu , Xianghong Guan , Hua Huang , Zemin Li , Guanglei Qiu , Chaohai Wei
|
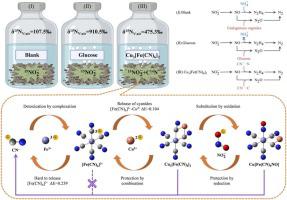
Cyanide is a typical toxic reducing agent prevailing in wastewater with a well-defined chemical mechanism, whereas its exploitation as an electron donor by microorganisms is currently understudied. Given that conventional denitrification requires additional electron donors, the cyanide and nitrogen can be eliminated simultaneously if the reducing HCN/CN and its complexes are used as inorganic electron donors. Hence, this paper proposes anaerobic cyanides oxidation for nitrite reduction, whereby the biological toxicity and activity of cyanides are modulated by bimetallics. Performance tests illustrated that low toxicity equivalents of iron-copper composite cyanides provided higher denitrification loads with the release of cyanide ions and electrons from the complex structure by the bimetal. Both isotopic labeling and Density Functional Theory (DFT) demonstrated that CN-N supplied electrons for nitrite reduction. The superposition of chemical processes reduces the biotoxicity and enhances the biological activity of cyanides in the CN/Fe/Cu/ coexistence system, including complex detoxification of CN by Fe, CN release by Cu from [Fe(CN)], and NO release by nitrite substitution of −CN groups. Cyanide is the smallest structural unit of C/N-containing compounds and serves as a probe to extend the electron-donating principle of anaerobic cyanides oxidation to more electron-donor microbial utilization.
中文翻译:

双金属调节生物毒性和亚硝酸盐还原活性的厌氧氰化物氧化
氰化物是废水中普遍存在的一种典型的有毒还原剂,具有明确的化学机制,而微生物对其作为电子供体的利用目前尚未得到充分研究。鉴于传统反硝化需要额外的电子给体,如果使用还原性HCN/CN及其络合物作为无机电子给体,可以同时消除氰化物和氮气。因此,本文提出厌氧氰化物氧化用于亚硝酸盐还原,从而通过双金属来调节氰化物的生物毒性和活性。性能测试表明,铁铜复合氰化物的低毒当量通过双金属从复杂结构中释放氰化物离子和电子,提供了更高的反硝化负荷。同位素标记和密度泛函理论 (DFT) 都证明 CN-N 为亚硝酸盐还原提供电子。化学过程的叠加降低了CN/Fe/Cu/共存体系中氰化物的生物毒性并增强了其生物活性,包括Fe对CN的复杂解毒、Cu从[Fe(CN)]中释放CN、以及由[Fe(CN)]中的Cu释放NO。 -CN 基团的亚硝酸盐取代。氰化物是含C/N化合物的最小结构单元,可作为探针将厌氧氰化物氧化的供电子原理扩展到更多供电子微生物的利用。
更新日期:2024-05-07
中文翻译:

双金属调节生物毒性和亚硝酸盐还原活性的厌氧氰化物氧化
氰化物是废水中普遍存在的一种典型的有毒还原剂,具有明确的化学机制,而微生物对其作为电子供体的利用目前尚未得到充分研究。鉴于传统反硝化需要额外的电子给体,如果使用还原性HCN/CN及其络合物作为无机电子给体,可以同时消除氰化物和氮气。因此,本文提出厌氧氰化物氧化用于亚硝酸盐还原,从而通过双金属来调节氰化物的生物毒性和活性。性能测试表明,铁铜复合氰化物的低毒当量通过双金属从复杂结构中释放氰化物离子和电子,提供了更高的反硝化负荷。同位素标记和密度泛函理论 (DFT) 都证明 CN-N 为亚硝酸盐还原提供电子。化学过程的叠加降低了CN/Fe/Cu/共存体系中氰化物的生物毒性并增强了其生物活性,包括Fe对CN的复杂解毒、Cu从[Fe(CN)]中释放CN、以及由[Fe(CN)]中的Cu释放NO。 -CN 基团的亚硝酸盐取代。氰化物是含C/N化合物的最小结构单元,可作为探针将厌氧氰化物氧化的供电子原理扩展到更多供电子微生物的利用。































 京公网安备 11010802027423号
京公网安备 11010802027423号